potassium iodide conductivity
You should explain this conductivity in terms of the free electrons within a metallic structure. The former was purified by several recrystallizations.

Temperature Dependence Of The Ionic Conductivity Of The Gel Polymer Download Scientific Diagram
Yes zinc chloride and potassium iodide.

. It also can conduct electrical energy when moltenhowever that is as a result of potassium ions moderately than the iodine ions. 58865 R5886500 Potassium Chloride Conductivity Standard 50 µScm at 25C. The only chemicals used in the research were potassium iodide and iodine.
Two regions A and B corresponding to different activation energy of conductivity are identified. 500 mL 1 L 4 L. Temp F µScm Acetaldehyde 59 17.
The conductivity reaches 366. The compound KAg 4 I 5 has an exceptionally high ionic conductivity for a solid reaching 031 ohm 1 cm 1 at the incongruent melting point. Conductivity Chart of Liquids conductivity too low for mag Low conductivity appl.
Potassium iodide is a component in the electrolyte of dyes sensitized solar cells DSSC along with iodine. Thermodynamic data derived from emf. Potassium Iodide 5 644 33800 10 68000 20 146000.
Potassium iodide resolution is ionic making it electrically conductive. The solid salt of potassium and iodine potassium iodide KI conducts poorly as do all salts which are ionic compounds. Potassium oxalate K2C2O4 16622 7 69.
One was the pure resublimed iodine prepared This lack of polarization is no evidence that the conductivity is. 588513 R5885130 Potassium Chloride Conductivity Standard 129 µScm at 25C. 5886 R5886000 Potassium Chloride Conductivity Standard 74 µScm at 25C.
Potassium nitrate KNO3 10110 7 68. Print Potassium Chloride Conductivity Standard 100 μScm at 25C Ricca Chemical. The increase in amorphous phase and free K ion concentration was responsible for the conductivity improvement in the electrolyte system.
However when in a solid dry form the compound does not conduct electricity. Potassium sulfide K2 74. Potassium permanganate KMnO4 15804 4 70.
Ionic conductivity of pure and doped KI is measured in the temperature range 200 to 700c. Potassium Chloride Conductivity Standard 100 μScm at 25C Ricca Chemical Structure Search. The measurements were performed in.
Potassium sulfate K2SO4 17426 4 73. Two kinds of iodine were used. Offices at 1-866-404-5415 for assistance.
The electrical conductivity of pure KI and CdI₂-doped KI has been studied in the temperature range 200 to 23C. Please contact the SmartMeasurement factory for assistance. Electrical conductivity is denoted by the Greek letter σ sigma and its measured.
The solubility of Ba 2 in KI is much higher than that of Cd 2 Pb 2 and Ca 2. Electrical conductivity of potassium iodide between 200 C and room temperature Prasad Mahendra Abstract. All the metals conduct electricity well.
We find that the surface states are passivated apart from the modified lattice structure. Name by Wt. A large contribution to the conductivity by the anion vacancies is observed in pure KI at high temperatures.
Measurements indicate that the formation of KAg 4 I 5 from K 2 AgI 3 and AgI is accompanied by an entropy gain implying. It can also conduct electricity when molten but this is due to the potassium ions rather than the iodine ions. 228 rows Conductivity values displayed in yellow are questionable.
Potassium iodide Kl 16603 14 67. Here we systematically investigate the regulatory mechanisms of the performance of perovskites by exploiting potassium iodide KI doping. A significant increase in the conductivity and radiative efficiency is achieved under such conditions.
588680 R5886800 Potassium Chloride Conductivity Standard 80 µScm at 25C. Potassium phosphate monobasic KH2PO4 13613 4 71. If a particular chemical is not listed or if you would like application assistance with an electromagnetic flowmeter application please feel free to contact SmartMeasurement TM s US.
Platinous iodide I2Pt CID 139965 - structure chemical names physical and chemical properties classification patents literature biological activities safety. This happens once the solid has been. However when in a strong dry type the compound doesnt conduct electrical energy.
Potassium iodide solution is ionic making it electrically conductive. Electrical conductivity is denoted by the Greek letter σ. Potassium phosphate dibasic K2HPO4 17418 4 72.
Graphical abstract The ionic conductivity of solid polymer electrolytes PVCPEOKI 42542515 is 366 10 4 S cm 1 at ambient temperature with an ionic transference number of 098 which is. Standard aqueous solution of potassium chloride or potassium iodide. The current is carried by the Ag ions.
Some substances are ionic but electrical conduction is only possible when the ions are free and mobile. The activity of a salt in an electrolyte has a direct impact on the performance of electrochemical cells because it can affect both the potential between phases and transport through the electrolyte. Click to view available options Quantity.
No none of them. The direct current conductivity of DMSO KI solutions was determined with the use of the impedance spectroscopy.

Pdf Electrical Conductivity Of Potassium Iodide Between 200 C And Room Temperature

Why Does Potassium Iodide Solution Conduct Electricity

Pdf Electrical Conductivity Of Potassium Salt Dimethylsulfoxide Water Systems At Different Temperatures Semantic Scholar

Potassium Iodide Conductivity Youtube

Ionic Conductivity And Dielectric Constant Data Of A Agarose With Ki Download Scientific Diagram

Why Does Potassium Iodide Solution Conduct Electricity

Pdf Electrical Conductivity Of Potassium Iodide Between 200 C And Room Temperature

Pdf Electrical Conductivity Of Potassium Iodide Between 200 C And Room Temperature
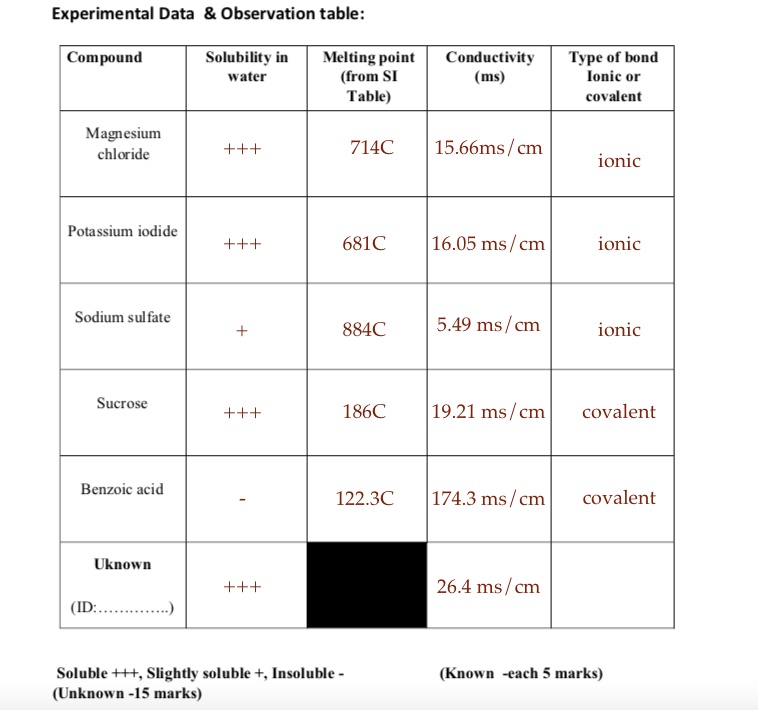
Solved Experimental Data Observation Table Compound Solubility In Water Melting Point From Si Table Conductivity Ms Type Of Bond Lonic Or Covalent Magesium Chloride 714c 15 66ms Cm Ionic Potassium Iodide 681c

Why Does Potassium Iodide Solution Conduct Electricity
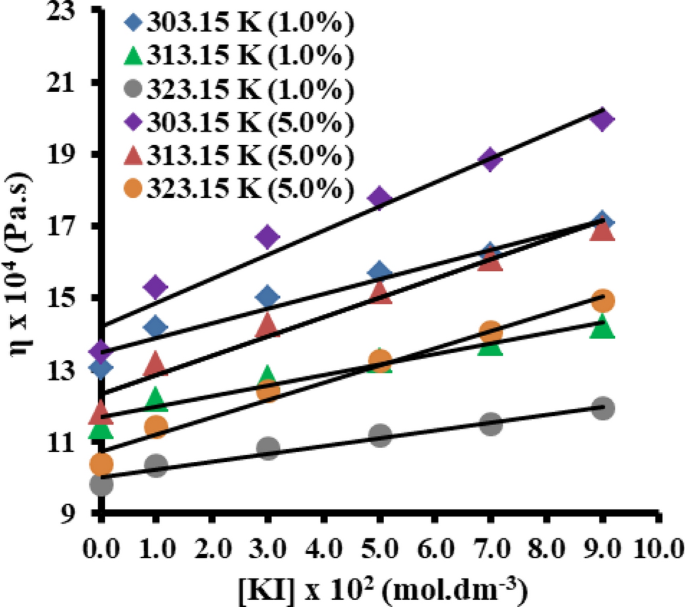
The Effect Of Potassium Iodide On The Viscosity Of Vegetable Oil N N Dimethylformamide Solvent At Different Temperatures Springerlink

Pdf Electrical Conductivity Of Potassium Iodide Between 200 C And Room Temperature

Solved D What Ions If Any Do Each Of The Following Chegg Com
Solutions Of Potassium And Iodide In Water And Solution Of Lead Nitrate In Water Being Mixed Together Turning Yellow And Forming A Lead Iodide Stock Photo Alamy

Pdf Ionic Interaction Of Potassium Iodide In Edible Oils Dmf System By Viscosity Method Semantic Scholar

Why Does Potassium Iodide Solution Conduct Electricity

Effects Of Potassium Iodide Ki On Crystallinity Thermal Stability And Electrical Properties Of Polymer Blend Electrolytes Pvc Peo Ki Sciencedirect

Potassium Iodide Ki Optical Material

Pdf Ionic Interaction Of Potassium Iodide In Edible Oils Dmf System By Viscosity Method Semantic Scholar
Comments
Post a Comment